Clariant presents its active healthcare packaging solutions for Chinas pharmaceutical and healthcare industry
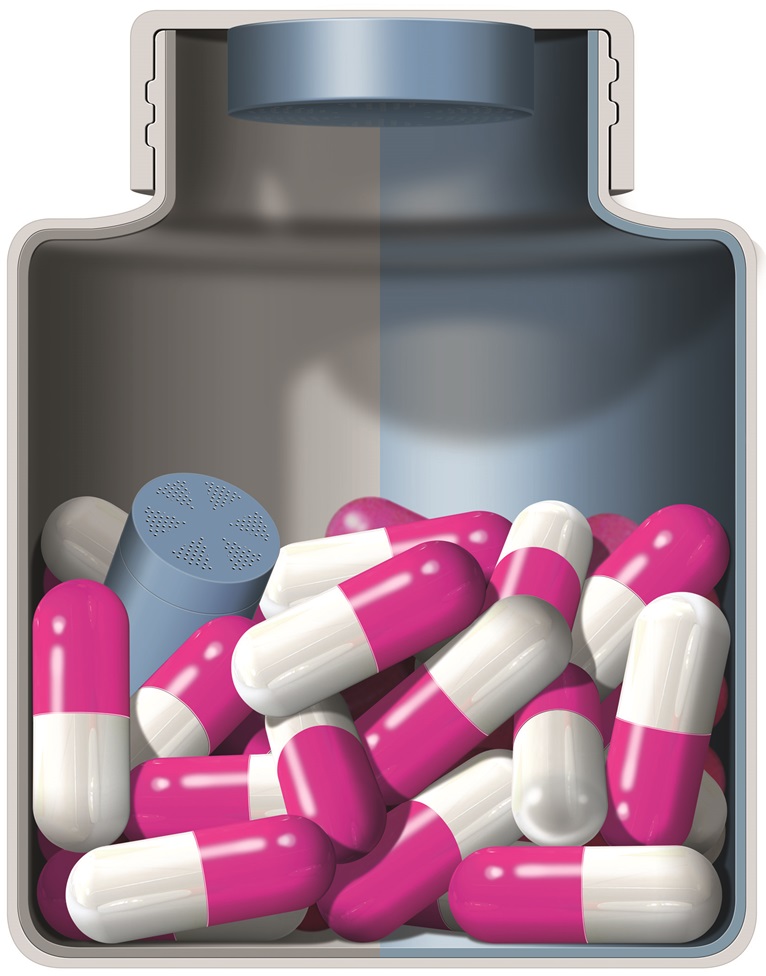
- Full line of desiccant canisters to be featured at 75th API China & PHARMPACK & SINOPHEX exhibition
- Successful acquisition and system integration of healthcare packaging company VitaPac has strengthened Clariant's production capacity, development level and services
The pharmaceutical and healthcare industry of China, despite of China's weak macro economy, is still growing sustainedly and quickly at 10%-15% annually. One of the biggest challenges met by the growing industry is a secured means of storage for the proper protection of healthcare products. Even within plastic containers, tablets and pills can still be exposed to moisture and oxygen that permeate the package from the outside environment. It does not only affect the chemical and physical stability of drugs but could also leave undesirable odors.
To overcome such challenge for the pharmaceutical industry, seasoned healthcare packaging solution providers like Clariant are supplying the industry with desiccants and systems designed especially for the protection of drugs. They preserve drug products by addressing the moisture that can enter the microenvironment of the package from various sources, including moisture trapped in the headspace during packaging or released from the drug product itself, moisture vapor transmission through pharmaceutical bottle walls throughout shelf life and ingress during open-close cycles by the patient, thus ensuring the dry and protective atmosphere durring the "full use cycle" of the drug product.
Desiccant canisters, small cylindrical containers filled with active agents, can be inserted at high rates of speed into pharmaceutical bottles and have become the gold standard of pharmaceutical desiccants. Clariant's full line of rigid desiccant canisters lead the industry when controlled atmosphere packaging is required to control moisture, oxygen, odor and other volatiles. The active packaging platform is customizable with various sorbent materials. Ideal for pharmaceutical packaging and healthcare products packaging, they are available with silica gel, bentonite clay, molecular sieve and oxygen absorbing material.
Clariant has strengthened its production capacity, development level and services after acquiring the former VitaPac last year, which has been fully integrated into Clariant's pharmaceutical packaging business line. Through intergation and synergy with VitaPac, Clariant's expertise and capability in delivering healthcare packaging solution was strengthened, complementing the portfolio, reducing the delivery cycle, increasing the capacity, and enlarging the local R&D team. All this has made it possible for Clariant to supply to its customers in the fast-growing healthcare industry of China as well as other regions globally.
"Our customers find Canisters a proven and growing form of moisture control for pharmaceutical applications, which can be tailored in different combinations for specific needs and humidity levels. Our newly acquired production facility and R&D team in Dongguan has made the products even more accessible for those customers in the Greater China region, especially when customization is needed," explains Albert Zhao, Head of Sales Asia Pacific and Operation China, Clariant Healthcare Packaging.
Clariant's desiccant canisters and other products meet the safety standards of food and drug administrations in the US, EU and China for direct contact with food and drugs as well as China's pharmaceutical packaging material license. In addition, Clariant offers desiccants that are integrated into product packaging in three different ways: dropped-in; integrated into a closure or in the bottom of the container; or embedded directly into the polymer matrix of the container or another packaging component.
During the 3-day show at API China & PHARMPACK & SINOPHEX, Clariant will showcase its products and solutions in Hall 5 at Booth No.5A55. Clariant's market sales and technical R&D teams will be available on-site to explain products in detail and to answer any question from the visitors.

